-
ishvartadvi01@ymail.com -
+91-7016332396
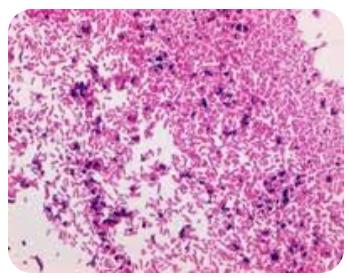
Safranin 0 Supplier
Contact Us
Arti Chemicals
Plot No. 480/1, Phase-2, GIDC, Vatva, Vatva, Ahmedabad, Gujarat - 382445, India
Mobile : +91-7016332396
In order to produce quality products, we make use of superior grade raw materials. Our quality auditors strictly check the raw materials against varied quality parameters before procurement. Be it any place or time; we make on time deliveries of the goods to the customers.
About Safranin
Safranines are the azonium compounds of symmetrical 2,8-dimethyl-3,7-diamino-phenazine. They are obtained by the joint oxidation of one molecule of a para-diamine with two molecules of a primary amine; by the condensation of para-aminoazo compounds with primary amines, and by the action of para-nitrosodialkylanilines with secondary bases such as diphenylmetaphenylenediamine. They are crystalline solids showing a characteristic green metallic lustre; they are readily soluble in water and dye blue or violet. They are strong bases and form stable monacid salts. Their alcoholic solution shows a yellow-red fluorescence.
Phenosafranine is not very stable in the free state; its chloride forms green plates. It can be readily diazotized, and the diazonium salt when boiled with alcohol yields aposafranine or benzene induline, C18H12N3. F. Kehnnann showed that aposafranine could be diazotized in the presence of cold concentrated sulfuric acid, and the diazonium salt on boiling with alcohol yielded phenylphenazonium salts. Aposafranone, C18H12N20, is formed by heating aposafranine with concentrated hydrochloric acid. These three compounds are perhaps to be represented as ortho- or as para-quinones.
The "safranine" of commerce is a ortho-tolusafranine. The first aniline dye-stuff to be prepared on a manufacturing scale was mauveine, which was obtained by Sir William Henry Perkin by heating crude aniline with potassium bichromate and sulfuric acid. Mauveine was converted to parasafranine (1,8-dimethyl Safranine) by Perkin in 1878 by oxidative/reductive loss ofthe 7-N-para-tolyl group.[2]
Specifications
| Chemical Name | 3,7- Diamino - 2, 8-dimethyl-5-phenyl-phenazinium chloride |
| C.A.S. NO | 477-73-6 |
| C.I.No. | 50240 |
| M.F. | C20H19N4Cl |
| M.W. | 350.85 gm/mole |
| Appearance | Dark Green Crystalline Powder |
| Dye Content (Spectrophotometric) | > 98% |
| Solubility 0.1% (in water) | Clear orange red Solution with fluorescence |
| Absorption maximum (in water)λmax | 530-534 nm |
| Absorptivity (A1% / 1cm in water at λmax) | > 1450 |
| Loss on drying (1100C) | < 5% |